Coal is a fossil fuel extracted from the ground by underground mining or open-pit mining ( surface mining). Often associated with the Industrial Revolution, coal remains an enormously important fuel and is the largest single source of electricity world-wide.
Properties[]
It is a readily combustible black or brownish-black sedimentary rock.

Coal
Composition[]
It is composed primarily of carbon along with assorted other elements, including Sulfur. Carbon forms more than 50 percent by weight and more than 70 percent by volume of coal (this includes inherent moisture). This is dependent on coal rank, with higher rank coals containing less hydrogen, oxygen and nitrogen, until 95% purity of carbon is achieved at Anthracite rank and above. Graphite formed from coal is the end-product of the thermal and diagenetic conversion of plant matter (50% by volume of water) into pure carbon.
Coal usually contains a considerable amount of incidental moisture, which is the water trapped within the coal in between the coal particles. Coals are usually mined wet and may be stored wet to prevent spontaneous combustion, so the carbon content of coal is quoted as both a 'as mined' and on a 'moisture free' basis.
Lignite and other low-rank coals still contain a considerable amount of water and other volatile components trapped within the particles of the coal, known as its macerals. This is present either within the coal particles, or as hydrogen and oxygen atoms within the molecules. This is because coal is converted from carbohydrate material such as cellulose, into carbon, which is an incremental process (see below). Therefore coal carbon contents also depend heavily on the degree to which this cellulose component is preserved in the coal.
Other constituents of coals include mineral matter, usually as silicate minerals such as clays, illite, kaolinite and so forth, as well as carbonate minerals like siderite, calcite and aragonite. Iron sulfide minerals such as pyrite are common constituents of coals. Sulfate minerals are also found, as is some form of salt, trace amounts of metals, notably iron, uranium and cadmium, and rarely gold.
Methane gas is another component of coal, produced not from bacterial means but from methanogenesis. Methane in coal is dangerous as it can cause coal seam explosions especially in underground mines, and may cause the coal to spontaneously combust. It is, however, a valuable by-product of some coal mining, serving as a significant source of natural gas.
Coal composition is determined by specific coal assay techniques, and is performed to quantify the physical, chemical and mechanical behaviour of the coal, including whether it is a good candidate for coking coal.
Types of coal[]
As geological processes apply pressure to peat over time, it is transformed successively into:
- Lignite - also referred to as brown coal, is the lowest rank of coal and used almost exclusively as fuel for steam-electric power generation. Jet is a compact form of lignite that is sometimes polished and has been used as an ornamental stone since the Iron Age.
- Sub-bituminous coal - whose properties range from those of lignite to those of bituminous coal and are used primarily as fuel for steam-electric power generation.
- Bituminous coal - a dense coal, usually black, sometimes dark brown, often with well-defined bands of bright and dull material, used primarily as fuel in steam-electric power generation, with substantial quantities also used for heat and power applications in manufacturing and to make coke.
- Anthracite - the highest rank, used primarily for residential and commercial space heating.
Coal as fuel[]
- See also Clean coal
Coal is primarily used as a solid fuel to produce heat through combustion.
World coal consumption is about 5,800 million short tons (5.3 petagrams) annually, of which about 75% is used for electricity production. The region including China and India uses about 1,700 million short tons (1.5 Pg) annually, forecast to exceed 3,000 million short tons (2.7 Pg) in 2025. The USA consumes about 1,100 million short tons (1.0 Pg) of coal each year, using 90% of it for generation of electricity. Coal is the fastest growing energy source in the world, with coal use increasing by 25% for the three-year period ending in December 2004 (BP Statistical Energy Review, June 2005).
When coal is used in electricity generation, it is generally pulverized and then burned (~35% efficient). The heat produced is used to create steam, which is then used to spin turbines which turn generators and create electricity. Approximately 40% of the Earth's current electricity production is powered by coal, and the total known deposits recoverable by current technologies are sufficient for 300 years' use at current rates (see World Coal Reserves, below).
A promising, more energy efficient way of using coal for electricity production would be via solid-oxide fuel cells or molten-carbonate fuel cells (or any oxygen ion transport based fuel cells that do not discriminate between fuels, as long as they consume oxygen), which would be able to get 60%-85% combined efficiency (direct electricity + waste heat steam turbine), compared to 30-40% currently possible with only steam turbines. Currently these fuel cell technologies can only process gaseous fuels, and they are also sensitive to sulfur poisoning, issues which would first have to be worked out before large scale commercial success is possible with coal. As far as gaseous fuels go, one idea is pulverized coal in a gas carrier (nitrogen), especially if the resulting carbon dioxide is sequestered, and has to be separated anyway from the carrier. A better idea is coal gasification with water, then the water recycled.
Gasification[]
High prices of oil and natural gas are leading to increased interest in "BTU Conversion" technologies such as coal gasification, methanation, liquefacation, and solidification.
In the past, coal was converted to make coal gas, which was piped to customers to burn for illumination, heating, and cooking. At present, the safer natural gas is used instead. South Africa still uses gasification of coal for much of its petrochemical needs.
Gasification is also a possibility for future energy use, as it generally burns hotter and cleaner than conventional coal and can thus spin a more efficient gas turbine rather than a steam turbine. This is used in IGCC power plants. It also makes for the possibility of zero carbon dioxide emissions even though the energy comes from the conversion of carbon to carbon dioxide. This is because gasification produces a much higher concentration of carbon dioxide than direct combustion of coal in air (which is mostly nitrogen). The higher concentrations of carbon dioxide makes carbon capture and storage more economical than otherwise.
Liquefaction[]
Coal can also be converted into liquid fuels like gasoline or diesel by several different processes. The Fischer-Tropsch process of indirect synthesis of liquid hydrocarbons was used in Nazi Germany, and for many years by Sasol in South Africa - in both cases, because those regimes were politically isolated and unable to purchase crude oil on the open market. Coal would be gasified to make syngas (a balanced purified mixture of CO and H2 gas) and the syngas condensed using Fischer-Tropsch catalysts to make light hydrocarbons which are further processed into gasoline and diesel. Syngas can also be converted to methanol: which can be used as a fuel, fuel additive, or further processed into gasoline via the Mobil M-gas process.
A direct liquefaction process Bergius process (liquefaction by hydrogenation) is also available but has not been used outside Germany, where such processes were operated both during World War I and World War II. SASOL in South Africa has experimented with direct hydrogenation. Several other direct liquefaction processes have been developed, among these being the SRC-I and SRC-II (Solvent Refined Coal) processes developed by Gulf Oil and implemented as pilot plants in the United States in the 1960's and 1970's.[1]
Yet another process to manufacture liquid hydrocarbons from coal is low temperature carbonization (LTC). Coal is coked at temperatures between 450 and 700 °C compared to 800 to 1000 °C for metallurgical coke. These temperatures optimize the production of coal tars richer in lighter hydrocarbons than normal coal tar. The coal tar is then further processed into fuels. The process was developed by Lewis Karrick, an oil shale technologist at the U.S. Bureau of Mines in the 1920s.[2]
All of these liquid fuel production methods release carbon dioxide (CO2) in the conversion process, far more than is released in the extraction and refinement of liquid fuel production from petroleum. If these methods were adopted to replace declining petroleum supplies carbon dioxide emissions would be greatly increased on a global scale. For future liquefaction projects, Carbon dioxide sequestration is proposed to avoid releasing it into the atmosphere. As CO2 is one of the process streams, sequestration is easier than from flue gases produced in combustion of coal with air, where CO2 is diluted by nitrogen and other gases. Sequestration will, however, add to the cost.
Coal liquefaction is one of the backstop technologies that will limit escalation of oil prices and mitigate the alleged effects of peak oil. Estimates of the cost of producing liquid fuels from coal suggest that domestic U.S. production of fuel from coal becomes cost-competitive with oil priced at around 35 USD per barrel [3], (break-even cost), which is well above historical averages - but is now viable due to the spike in oil prices in 2004-2005. [4].
Among commercially mature technologies, advantage for indirect coal liquefaction over direct coal liquefaction are reported by Williams and Larson (2003). Estimates are reported for sites in China where break-even cost for coal liquefaction may be in the range between 25 to 35 USD/barrel of oil.
Coking and use of coke[]
- Main article: Coke (fuel)
Coke is a solid carbonaceous residue derived from low-ash, low-sulfur bituminous coal from which the volatile constituents are driven off by baking in an oven without oxygen at temperatures as high as 1,000 °C (2,000 °F) so that the fixed carbon and residual ash are fused together. Coke is used as a fuel and as a reducing agent in smelting iron ore in a blast furnace. Coke from coal is grey, hard, and porous and has a heating value of 24.8 million Btu/ton (29.6 MJ/kg). Byproducts of this conversion of coal to coke include coal-tar, ammonia, light oils, and "coal-gas".
Petroleum coke is the solid residue obtained in oil refining, which resembles coke but contains too many impurities to be useful in metallurgical applications.
Harmful effects of coal burning[]
Combustion of coal, like any other compound containing carbon, produces carbon dioxide (CO2), along with varying amounts of sulfur dioxide (SO2) depending on where it was mined. Sulfur dioxide reacts with water to form sulfurous acid. If sulfur dioxide is discharged into the atmosphere, it reacts with water vapor and is eventually returned to the Earth as acid rain.
Emissions from coal-fired power plants represent the largest source of carbon dioxide emissions, a primary cause of global warming. Many other pollutants are present in coal power station emissions. Some studies claim that coal power plant emissions are responsible for tens of thousands of premature deaths annually in the United States alone. Modern power plants utilize a variety of techniques to limit the harmfulness of their waste products and improve the efficiency of burning, though these techniques are not widely implemented in some countries, as they add to the capital cost of the power plant. To eliminate CO2 emissions from coal plants, carbon capture and storage has been proposed but is not yet in use.
Coal also contains many trace elements, including arsenic and mercury, which are dangerous if released into the environment. Coal also contains low levels of uranium, thorium, and other naturally-occurring radioactive isotopes whose release into the environment may lead to radioactive contamination.[5][6] While these substances are trace impurities, enough coal is burned that significant amounts of these substances are released, paradoxically resulting in more radioactive waste than nuclear power.
Coal fires[]
There are hundreds of coal fires burning around the world.[7] Those burning underground can be difficult to locate and many can not be extinguished. Fires can cause the ground above to subside, combustion gases are dangerous to life, and breaking out to the surface can initiate surface wildfires.
Coal seams can be set on fire by spontaneous combustion or contact with a mine fire or surface fire. A grass fire in a coal area can set dozens of coal seams on fire.[8] [9] Coal fires in China burn 120 million tons of coal a year, emitting 360 million metric tons of carbon dioxide. This amounts to 2-3% of the annual worldwide production of CO2 from fossil fuels, or as much as emitted from all of the cars and light trucks in the United States.
World coal reserves[]
It has been estimated that, as of 1996, there is around one exagram (1 × 1015 kg) of total coal reserves accessible using current mining technology, approximately half of it being hard coal. The energy value of all the world's coal is well over 100,000 quadrillion Btu (100 zettajoules). There probably is enough coal to last for 300 years. However, this estimate assumes no rise in population, and no increased use of coal to attempt to compensate for the depletion of natural gas and petroleum. A recent (2003) study by scientist Gregson Vaux, which takes those factors into account, estimates that coal could peak in the United States as early as 2046, on average. "Peak" does not mean coal will disappear, but defines the time after which no matter what efforts are expended coal production will begin to decline in quantity and energy content. The disappearance of coal will occur much later, around the year 2267, assuming all other factors do not change, which they naturally will.[10] British Petroleum, in its annual report 2005, estimated at 2004 end, there were 909,064 million tons of proved coal reserves worldwide, or 164 years reserve to production ratio.
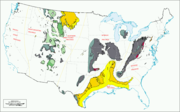
US coal regions.
The United States Department of Energy uses estimates of coal reserves in the region of 1,081,279 million short tons, which is about 4,786 BBOE (billion barrels of oil equivalent) [11]. The amount of coal burned during 2001 was calculated as 2.337 GTOE (gigatonnes of oil equivalent), which is about 46 MBOED (million barrels of oil equivalent per day) [12]. At that rate those reserves will last 285 years. As a comparison natural gas provided 51 MBOED, and oil 76 MBD (million barrels per day) during 2001.
See also[]
- Coal dust
- Coal mining techniques
- Coal-tar
- Energy value of coal
- Fluidized bed combustion
- List of environment topics
- Major coal producing regions
External links[]
- MSNBC report on coal pollution health effects in the United States
- Clean coal technologies
- Use of coal gas in fuel cells
- Advanced methods of using coal (Japanese Coal Energy Center)
- Learnaboutcoal.org
- Coal Mine Fire
References[]
- Dan Rottenberg, In the Kingdom of Coal. A narrative history of the U.S. coal industry. (Routledge, 2003.)
| This page uses Creative Commons Licensed content from Wikipedia (view authors). | 
|