 A temperature-versus-entropy diagram for steam |
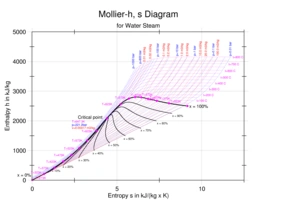
 A Mollier enthalpy-versus-entropy diagram for steam |
In engineering steam refers to vaporized water. In physical chemistry also it refers to vaporized water.
Properties[]
Steam is a pure, completely invisible gas (for mist see below), which at standard atmospheric pressure has a temperature of around 100 degrees Celsius, and occupies about 1,600 times the volume of liquid water (steam can of course be much hotter than the boiling point of water; such steam is usually called superheated steam).
In common speech, steam most often refers to the white mist that condenses above boiling water as the hot vapor ("steam" in the first sense) mixes with the cooler air. After gaseous steam has intermixed with air, it is no longer properly called steam and is instead referred to as water vapor.
Steam is a capacious reservoir for energy because of water's high heat of vaporization.
The International Association for the Properties of Water and Steam (IAPWS), maintains international-standard correlations for the thermodynamic [1] properties of steam, including IAPWS-IF97 (for use in industrial simulation and modelling) and IAPWS-95 (a general purpose and scientific correlation).
How produced[]
When the liquid, water comes in contact with a very hot liquid substance (such as lava, or molten metal) it can flash into steam very quickly; this is called a steam explosion[2]. (Such an explosion was probably responsible for much of the damage in the Chernobyl accident [3] and for many so-called 'foundry accidents').
Steam is also produced in steam boilers for various uses and also as a by product in some industries.
Uses[]
A steam engine uses the expansion of steam to drive a piston or turbine and so to perform mechanical work. In other industrial applications steam is used as a repository of energy, which is introduced and extracted by heat transfer. The ability to return condensed steam as water-liquid to the boiler at high pressure with relatively little expenditure of pumping power is also an advantage. Engineers use an idealised thermodynamic cycle, the Rankine cycle, to model the behaviour of steam engines.
In many countries major portion of electric power is produced using steam as the working fluid, mainly by using steam turbines. Condensation of steam to water often occurs at the low-pressure end of a steam turbine by use of condensers, since this maximises the energy efficiency. However such wet-steam conditions have to be carefully controlled to avoid excessive blade erosion in steam turbines.
Steam is used in saunas and steam showers to produce warmth and therapeutic effects in human beings.
See also[]
- Steam turbines
- Steam locomotive
- Steam roller
- Mist [4]
| This page uses Creative Commons Licensed content from Wikipedia (view authors). | 
|